Inter Transition Metals / Anatomy Of The Periodic Table Chemistrybytes Com - This heat is used to produce the steam from the water.
Inter Transition Metals / Anatomy Of The Periodic Table Chemistrybytes Com - This heat is used to produce the steam from the water.. Whereas other inner transition metals are used in many things such as goggles, television screens and color computer monitors, in the steel industry to remove carbon from iron and steel, movie projectors, searchlights, lasers, and tinted sunglasses. Cerium is used to color glass for lamps and is also used in lighters to start fires. The transition metals are malleable (easily hammered into shape or bent). Fe 3 c, fe 7 c 3 and fe 2 c. The transition metals are the elements you normally think of when you imagine a metal.
These elements share properties in common with each other: The transition metals are malleable (easily hammered into shape or bent). The lanthanides (except for the synthetic element promethium), along with scandium (sc) and yttrium (y) are sometimes referred to as rare earth elements or rare earth metals (or just rare earths). They are the lanthanides, and the actinides. Remember that the configuration is reversed from the fill order—the 4 s filled before the 3 d begins.

The most common ones are uranium which is found in the earth, plutonium which is man made ,and cerium which is also found in the earth.
Whereas other inner transition metals are used in many things such as goggles, television screens and color computer monitors, in the steel industry to remove carbon from iron and steel, movie projectors, searchlights, lasers, and tinted sunglasses. They consist of the lanthanides and the actinides. The transition metals are the elements you normally think of when you imagine a metal. The transition metals may be subdivided according to the electronic structures of their atoms into three main transition series, called the first, second, and third transition series, and two inner transition series, called the lanthanoids and the actinoids. We have daily contact with many transition metals. This heat is used to produce the steam from the water. The key difference between transition metals and metalloids is that the transition metals are chemical elements having atoms with unpaired d electrons whereas metalloids are chemical elements having their properties between metals and nonmetals. They are excellent conductors of heat and electricity. The transition metals are malleable (easily hammered into shape or bent). These elements were sometimes called rare earth metals due to their extremely low natural occurrence. These elements share properties in common with each other: The elements are called transition metals because the english chemistry charles bury used the term in 1921 to describe the transition series of elements, which referred to the transition from an inner electron layer with a stable group of 8 electrons to one with 18 electrons or the transition from 18 electrons to 32. Home / uncategorized / inner transition metals
They are normally shown in two rows below all the other elements. The most common ones are uranium which is found in the earth, plutonium which is man made ,and cerium which is also found in the earth. Radioactive metals like uranium produce a lot of heat when they undergo nuclear fission. The periodic table of elements is composed of metals, nonmetals, and metalloids.chemical elements are categorized as metals if they have metallic properties such as malleability, good electrical conductivity, easily remove electrons, etc. This heat is used to produce the steam from the water.
The key difference between transition metals and metalloids is that the transition metals are chemical elements having atoms with unpaired d electrons whereas metalloids are chemical elements having their properties between metals and nonmetals.
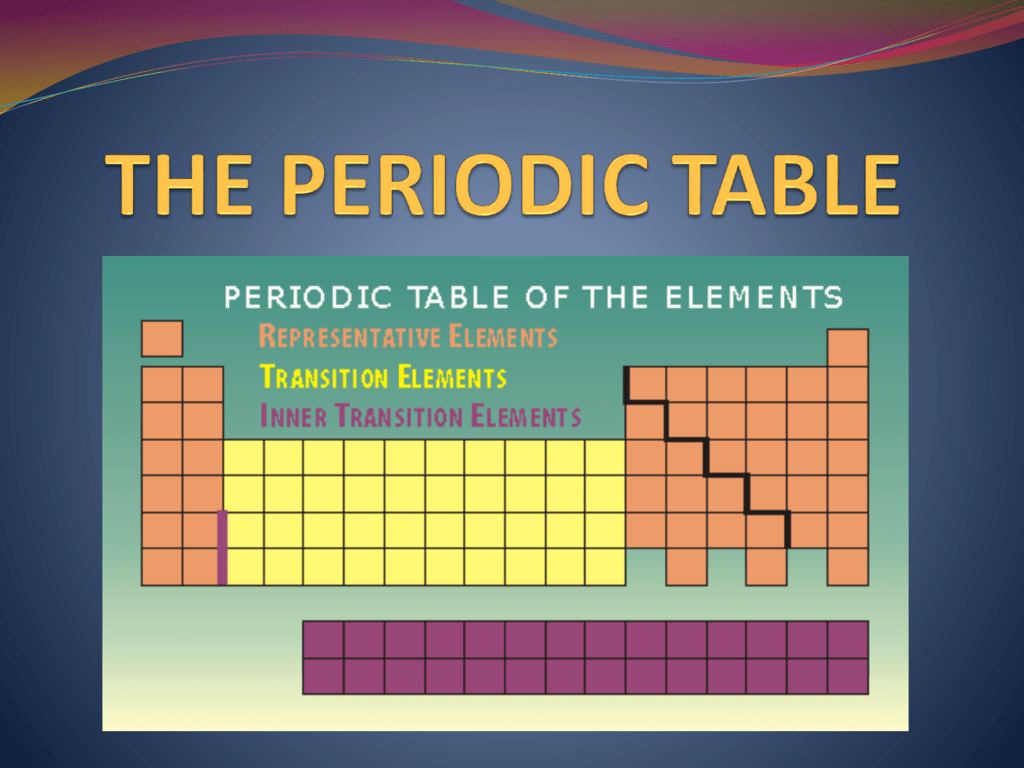
The periodic table of elements is composed of metals, nonmetals, and metalloids.chemical elements are categorized as metals if they have metallic properties such as malleability, good electrical conductivity, easily remove electrons, etc. The uses of inner transition metals are mentioned below. Radioactive metals like uranium produce a lot of heat when they undergo nuclear fission. For example, iron forms a number of carbides: The transition metals may be subdivided according to the electronic structures of their atoms into three main transition series, called the first, second, and third transition series, and two inner transition series, called the lanthanoids and the actinoids. Uranium and plutonium are inner transition metals which are used for manufacturing nuclear weapons. Other transition metals in 1b except au, 2b, 6b except cr, 8b and the representative metals in 3a, 4a and 5a are mined in the form of sulfides. Fe 3 c, fe 7 c 3 and fe 2 c. We have daily contact with many transition metals. • inner transition metals have low availability than transition metals and hence called 'rare earth metals'. In chemistry, the term transition metal (or transition element) has three possible definitions: They are normally shown in two rows below all the other elements. Start studying inner transition metals.
Let's find out the names and properties of these metals through this sciencestruck article. It is regarded as one of the most important elements for the future of Learn vocabulary, terms, and more with flashcards, games, and other study tools. Iron occurs everywhere—from the rings in your spiral notebook and the cutlery in your kitchen to automobiles, ships, buildings, and in the hemoglobin in your blood. Radioactive metals like uranium produce a lot of heat when they undergo nuclear fission.
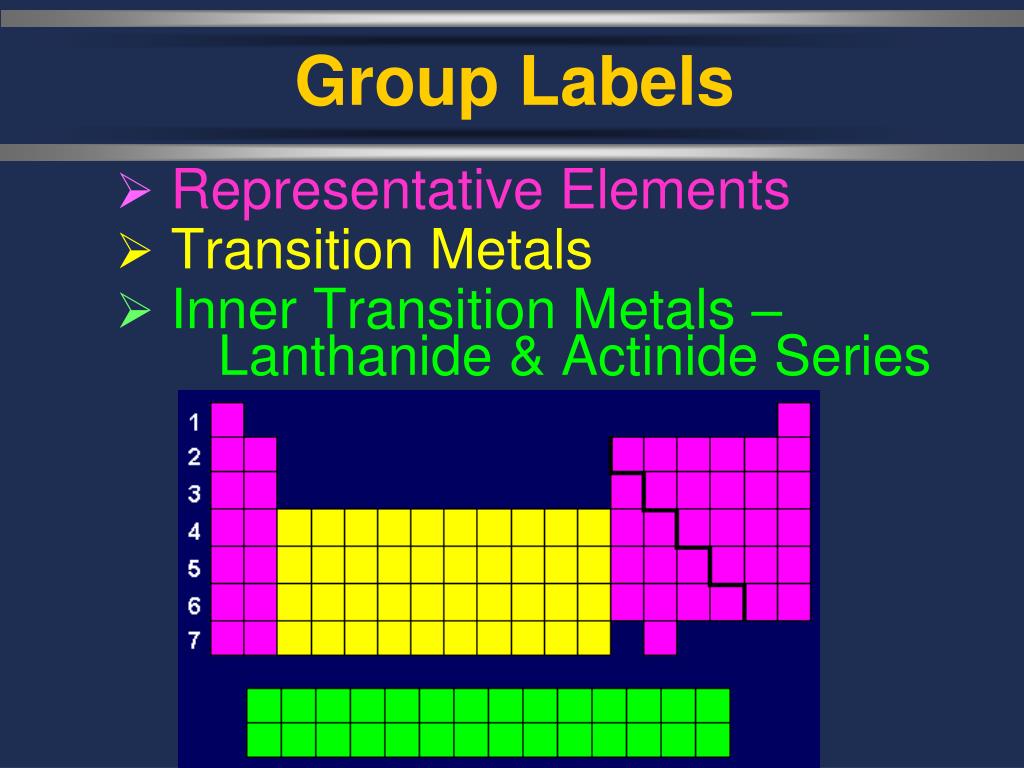
The inner transition metals are the two rows that are at the bottom of the periodic table.
Titanium is useful in the manufacture of lightweight, durable products such as bicycle frames, artificial hips, and jewelry. They are normally shown in two rows below all the other elements. Learn inner transition metals with free interactive flashcards. Transition metals in 4b, 5b, cr, mn, re plus a representative metal al are usually found in the form of oxides. The actinides are radioactive and mostly synthetic. Katie bruner cole shumway drake bishop mercedez cloninger lanthanide and actinide series uranium uranium is the heaviest and last naturally occurring element in the periodic table. Transition metals and inner transition metals are also metallic elements that are. Home / uncategorized / inner transition metals Transition elements (also known as transition metals) are elements that have partially filled d orbitals. The transition metals are the elements you normally think of when you imagine a metal. Compare and contrast the lanthanide series and the actinide series. • inner transition metals have low availability than transition metals and hence called 'rare earth metals'. These elements were sometimes called rare earth elements or rare earth metals due to their extremely low natural occurrence.
Komentar
Posting Komentar